ABCL Researchers Study Biological Control of the Southern Cattle Fever Tick
In September 2021, USDA ARS Australian Biological Control Laboratory (ABCL) staff members commenced an ancillary project to rear Encyrtid wasp parasitoids from the native 3-host tick species to assess their potential as biological control agents for the cattle fever tick, Rhipicephalus microplus.
Researchers from the ARS Cattle Fever Tick Research Laboratory in Edinburg, Texas, are investigating biocontrol solutions to this invasive tick that causes babesiosis in cattle resulting in huge economic losses in both milk and meat production. Genetic analyses suggest that R. microplus is a complex of five species/populations across its native range from Pakistan east into China and throughout Southeast Asia into Australia. The population in Texas was likely introduced from Southeast Asia. The genetics also suggest that the Australian species, described as R. australis, is more closely related to the Southeast Asian R. microplus than to other populations of R. microplus within this complex.
Encyrtidae wasps of the genus Ixodiphagus are specialist parasitoids of ticks throughout the world. Of the eight known species only two, I. texanus and I. hookeri, have been successfully colonized in the lab. Research suggests they have varying degrees of host-specificity and differ in which tick life stage(s) they attack. This variation in host preferences, between and within species, may well be habitat, host and/or climatically driven, but it may also be an indication of cryptic species.
In Australia, it is believed there are two Ixodiphagus species including I. hookeri, or a species morphologically similar, which was initially studied in southeast Queensland during the 1970s (Figure 1). That research revealed that the Queensland wasp parasitized two species of Haemaphysalis and two species of Ixodes ticks. Both the larvae and nymphs of Haemaphysalis bancrofti were parasitized, but in the other three species, only nymphs were attacked.

ABCL is conducting field surveys (Figure 2) of the three-host ticks native to southeast Queensland using a drag cloth and dry ice to attract questing ticks waiting in ground foliage. Depending on species, unfed ticks may only move small distances from where they have hatched (larvae) or dropped off a prior host (nymphs and adults). When conditions are favorable, they will extend their front legs until a passing animal is within reach. Their front legs have a sensory pit, the Haller’s organ, which can detect chemicals associated with mammals such as carbon dioxide, pheromones, and heat. Dry ice, as it releases carbon dioxide, is therefore often used as a trap to attract ticks for collection; calico and flannel sheets dragged across the ground and over low-lying foliage will mimic the movement of a passing animal. The ticks will attach to the sheet as they would to the hair of an animal. Since animals will often move in a group, repeating the cloth drag several times along the same transect will often retrieve additional ticks. Collected ticks are stored in low light, low temperature, and high humidity conditions where they can survive for many months without feeding.
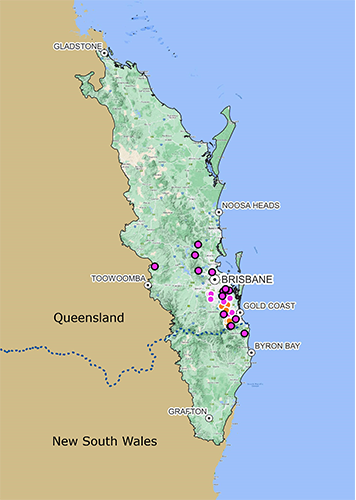
Often the same run at the same transect will retrieve different genera and species. Haemaphysalis ticks have been the most common retrieved, but Amblyomma and two species of Ixodes, the paralysis tick I. holocyclus, and I. tasmani, the common marsupial tick (Figure 3), have also been collected. One collection was also made from a dead bandicoot on the roadside in Cairns, north Queensland.

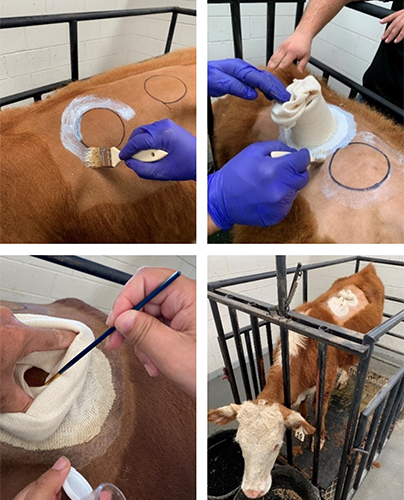
The ABCL’s next goal is to assess the presence and diversity of local Ixodiphagus parasitoids by engorging unfed ticks on cattle to rear the wasps. Colleagues at QAAFI (Queensland Alliance for Agriculture and Food Innovation, University of Queensland) will undertake this work at the Pinjarra Hills Research Precinct. They will use the rearing technique developed by the Cattle Fever Tick Research Laboratory in Texas (Figure 4): A stockinette is glued onto a shaven patch of the cow; the paper cup keeps the gauze from settling into the glue; it is removed after the glue dries; ticks are placed inside and the stockinette is tied to prevent escape.
Once engorged, the ticks will naturally fall off their host into the stockinette. ABCL will monitor the ticks for emergence of Ixodiphagus parasitoids. Adult parasitoids will be shipped to the Cattle Fever Tick Research Laboratory for evaluation. Where possible, genetic analysis of the ticks will also be conducted to develop an updated knowledge of the species diversity of this region and to further screen for evidence of Ixodiphagus DNA.
Although Rhipicephalus australis is not strictly native to Australia, it is naturalized, probably through the importation of cattle from Timor in the 1860s. In Queensland, it is present in the natural environment alongside native three-host ticks including species of Ixodes and Haemaphysalis. R. australis is primarily a parasite of cattle. However, it has also been recorded from a range of other hosts including marsupials. Thus, an updated knowledge of these local associations among the Australian ticks and their parasitoids may well provide valuable insights into the global efforts to utilize classical biocontrol for tick infestations. Incorporating genetics tools into this research may also resolve some of the current uncertainty surrounding the Ixodiphagus species in Australia.
Authors: Authors: Jeff Makinson and John Goolsby
Contact: Matthew Purcell
USDA ARS Australian Biological Control Laboratory (ABCL) is based at the Ecosciences Precinct in Brisbane, Australia, and is administered through a Specific Cooperative Agreement between ARS and Australia’s Federal research body, the Commonwealth Scientific and Industrial Research Organisation (CSIRO). The agreement has been a very productive, mutually beneficial, long-term collaborative relationship originating in 1985. Assisting with solving U.S. problems is reciprocated by ARS through supplying Australian scientists with biological control agents from the America’s to control invasive weeds in Australia.
