Cracking the Code of Marek's Disease |
|
|
|
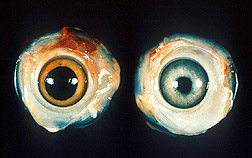
In an advance that could usher in new vaccines for protecting poultry, Agricultural Research Service (ARS) scientists have sequenced the genetic code of the chicken herpes virus that causes Marek's disease. Worldwide losses attributed to Marek's are an estimated $1 billion annually. The disease manifests as tumors on the bird's spleen, liver, lung, kidney, and other tissues. Other disease symptoms include neurological disorders, such as partial paralysis in the bird's legs or wings. Since the 1960s, poultry producers have used vaccines made from benign or "crippled" Marek's strains to immunize birds. But the virus has countered each new vaccine with ever more virulent strains, once again imperiling the industry. |
|
|
Now, with the virus's genetic code in hand, ARS researchers have begun studying the molecular mechanisms by which it causes disease. "Our aim is to create new vaccines through genetic engineering to protect poultry against hot [virulent] strains of Marek's disease viruses," says Sanjay M. Reddy, a medical veterinary officer at ARS' Avian Disease and Oncology Laboratory, in East Lansing, Michigan. Reddy's colleagues there are Robert F. Silva, Richard L. Witter, and Lucy F. Lee, who decoded the Marek's strain called MDV1-GA. Another ARS team, led by Daniel L. Rock and Gerald F. Kutish at the agency's Plum Island Animal Disease Center in New York, sequenced another MDV1 strain, described as Md5 vv (very virulent), and a harmless variant in turkeys called sterotype 3, which is used to vaccinate chickens.Mapping a Plan of Attack Using technology called a high-throughput sequencer, Rock's group decoded the order of 177,874 pairs of chemical "letters," called nucleotides, comprising the Md5 vv strain's DNA alphabet for 104 genes. "Having a pathogen's genetic blueprint is just like having the opposing team's playbook with its strategies for success; if you know what the game plan is, you can find ways to interfere with it," says Rock, who leads the center's African Swine Fever Research Unit. The advance could also give rise to new diagnostic tools for predicting where and when new strains emerge in the field and how virulent they are, says Rock. Located off Long Island, New York, the Plum Island lab's prime objective is decoding the genomics of exotic animal pathogens for diagnostics and vaccine development. Sequencing the two poultry viruses was a special assignment facilitated by the lab's high-throughput DNA sequencer and expertise in viral genomics. Using a different method, Lee's team charted a genetic stretch of MDV1-GA called the "unique long region," encompassing 113,508 nucleotide letters. "GA is a prototype of MDV that scientists all over the world have been working on for over 20 years," notes Lee. Both GA and Md5 strains of MDV1 are oncogenic (cancer-causing), and their sequences will reveal genes unique only to this group, she adds. Lee's colleague, ARS molecular biologist Silva, is now comparing the blueprints of these viruses with other herpesviruses. Such comparative studies could also yield more information about the virus's biological functions and could reveal specific genes that make particular MDV1 strains more virulent than others.
Cellular Saboteur The vaccines work by presenting the bird's immune system with an antigen, normally a protein, from a benign or crippled virus. This primes the bird's system to custom-make "killer cells" and antibodies that will mobilize specific attacks against virulent MDV1 strains. "You do need more than just vaccines," Witter acknowledges. "You need good management and good, resistant bird stocks. But without a vaccine, you'd be dead in the water. It can make the difference between 20 to 60 percent of birds dying in a layer flock, or not." Charles Beard, vice president of research and technical programs at the U.S. Poultry and Egg Association in Tucker, Georgia, agrees: "For poultry, that's 99.9 percent of the hope we have" against Marek's. Even with vaccines, the disease still costs the U.S. poultry industry millions of dollars annually in losses from bird deaths, diminished egg laying, and carcass condemnations at processing plants. Catch Me If You Can The emergence of new strains—such as Md5 vv—coupled with today's high-density poultry production, only exacerbates the problem. Beard puts it this way: "Over the years, these strains out in the field have gotten hotter and hotter, and we've run out of bullets." "It's been a cat-and-mouse game over the past 2 decades," adds Reddy. That's roughly when some of the earliest chicken vaccines began losing their effectiveness to more virulent MDV1 strains. Historically, scientists have produced the chicken vaccines by selecting strains either from field samples or lab research that, through natural mutations, replicate inside their host without causing serious harm. But finding suitable vaccine candidates can be a time-consuming, hit-or-miss affair. Witter notes, "Grow a virus long enough in an artificial system and it will mutate. If you catch them at the right time, these mutations can become useful in creating new vaccines." Now, with the letters of the virus's DNA alphabet spelled out, he adds, scientists can begin pinpointing specific genes of interest, such as those responsible for causing tumors in chickens. "It's like having a road map with street signs; you know exactly where you're going," says Lee, who works with Reddy and Silva on the recombinant vaccines. Cut and Paste One way scientists learn what the genes do is to delete, or "snip," them from the virus's DNA using enzymes. They then inoculate chickens with the genetically altered virus. This shows whether it will replicate, cause disease symptoms, or preferably stimulate an immune-system response. The Avian Disease and Oncology Laboratory has already applied the technique to study some of the genes involved in oncogenesis. "Once you understand how these genes work, you can design recombinant vaccines that protect against very virulent strains of MDV1," says Silva. Recombinant vaccines that show promise will be compared with commercial vaccines, he adds. If they still measure up, the lab will transfer the technology to a private company for further research and development. Even then, biotechnology's best chicken vaccine may not arm poultry farmers with the proverbial magic bullet. "You'd have to come up with a strategy that not only stops the virus from causing disease, but also keeps it from evolving so that it won't beat you again," Reddy ventures. Maybe the clue lies waiting, hidden within Marek's genetic code.—By Jan Suszkiw, Agricultural Research Service Information Staff. This research is part of Animal Health, an ARS National Program (#103) described on the World Wide Web at http://www.nps.ars.usda.gov. To reach scientists mentioned in this article, contact Jan Suszkiw, USDA-ARS Information Staff, 5601 Sunnyside Ave., Beltsville, MD 20705; phone (301) 504-1630, fax (301) 504-1641. |
|
"Cracking the Code of Marek's Disease" was published in the July 2001 issue of Agricultural Research magazine. |